The Electronic Common Technical Document (eCTD) is an international pharmaceutical specification to agency transfer of regulatory information. This document management system is hosted on an industry specific web application where modules are created for startups, small companies, and enterprises.
My role as a UX lead was to design an interactive tool based on a deep understanding of regulatory processes to facilitate the collection, revision and tracking of pharmaceutical documents for acceptance in different regions. This module increased the company’s customer base by ~500 users in the first quarter after shipping.
Over a period of 6 months, I performed the following tasks
- Engage in a weekly and bi-weekly in-depth discussion with industry experts to learn every regions’ regulatory standards and processes needed for drug compliance.
- Understand the role of the target audience to streamline their work.
-
Identify, sort and prioritize requirements based on what is most important:
Priority 1: Business Requirements
Example: “Attributes” are identifiers for pharmaceuticals and eCTD platforms require users to input them individually at every stage of the process. For the MVP of this product, I allowed users to input all attributes at one time which cascaded data to all sections of the module.
Priority 2: Business Enhancements
Example: Pre-populated attributes derived from regulatory standards for the user to select.
Priority 3: Product Enhancements
Example: Allowing users to edit pre-filled attributes from any place on the module which cascaded to all areas of the tool.
- Design and present working prototypes to companies and users to better highlight areas of success and adjustment before development handover.
- Finalize UI in development ready formats and present handover documentation.
The outcome of this research yielded a few key points:
- Regions are authorized to request revisions in a specified scope and every action made must be stored and retrievable at any moment.
- Pharmaceutical standards can differ wildly from one region to the next, however there are overlapping regulations that can be applied throughout all regions in the world.
- The principal user of this tool is a Regulatory Affairs Officer (RAO), who is performing functions such as uploading, modifying, evaluating and sorting necessary documentation. Secondary to that is the role of an Administrator who is managing all users, permissions and subscriptions.


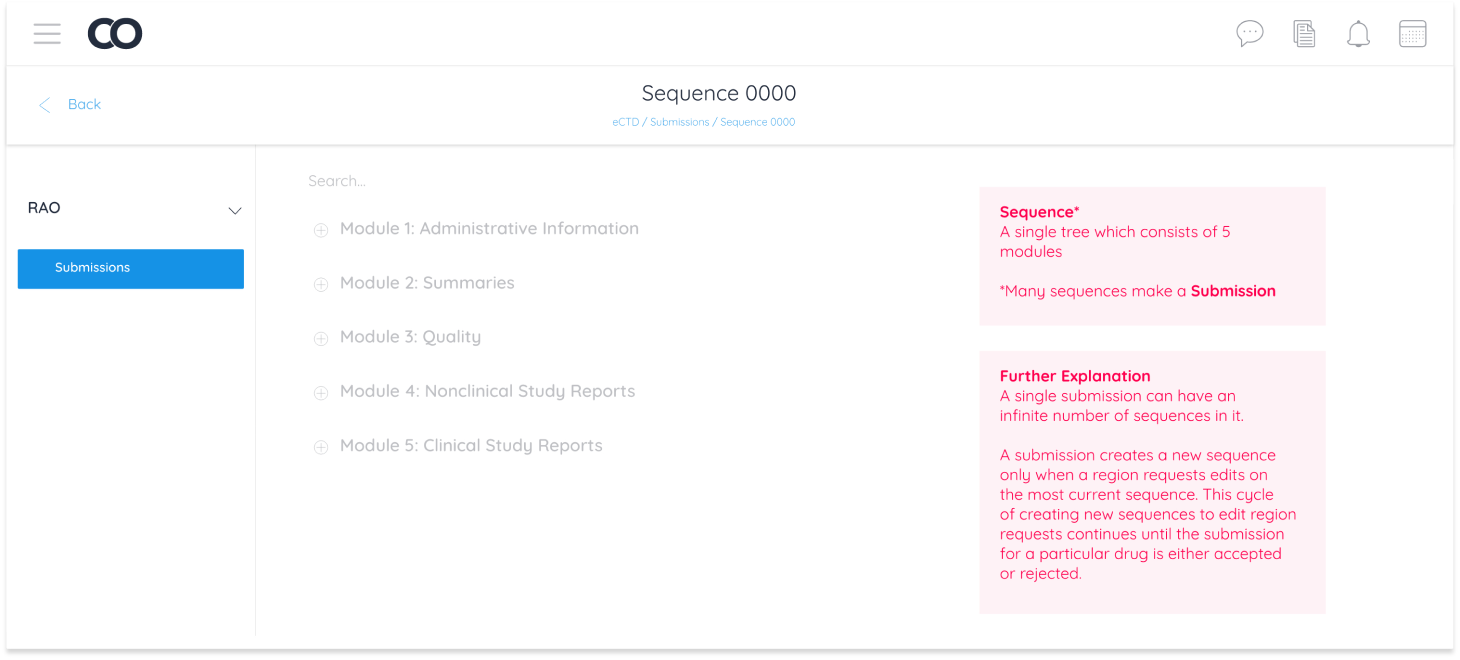
eCTD is a module with an extensive backlog of data storage, for this reason it was important to understand the relationship between users and the system. It was necessary for the system to store and retrieve the whole upload history and every action taken.
To achieve this, I created user-to-system workflows which included actions the user takes and the systems’ response. This gave clarity to the user experience in relation to the data.
I utilized hierarchy in industry language as the base for information architecture and communicated nuances and UX copy to the development team.
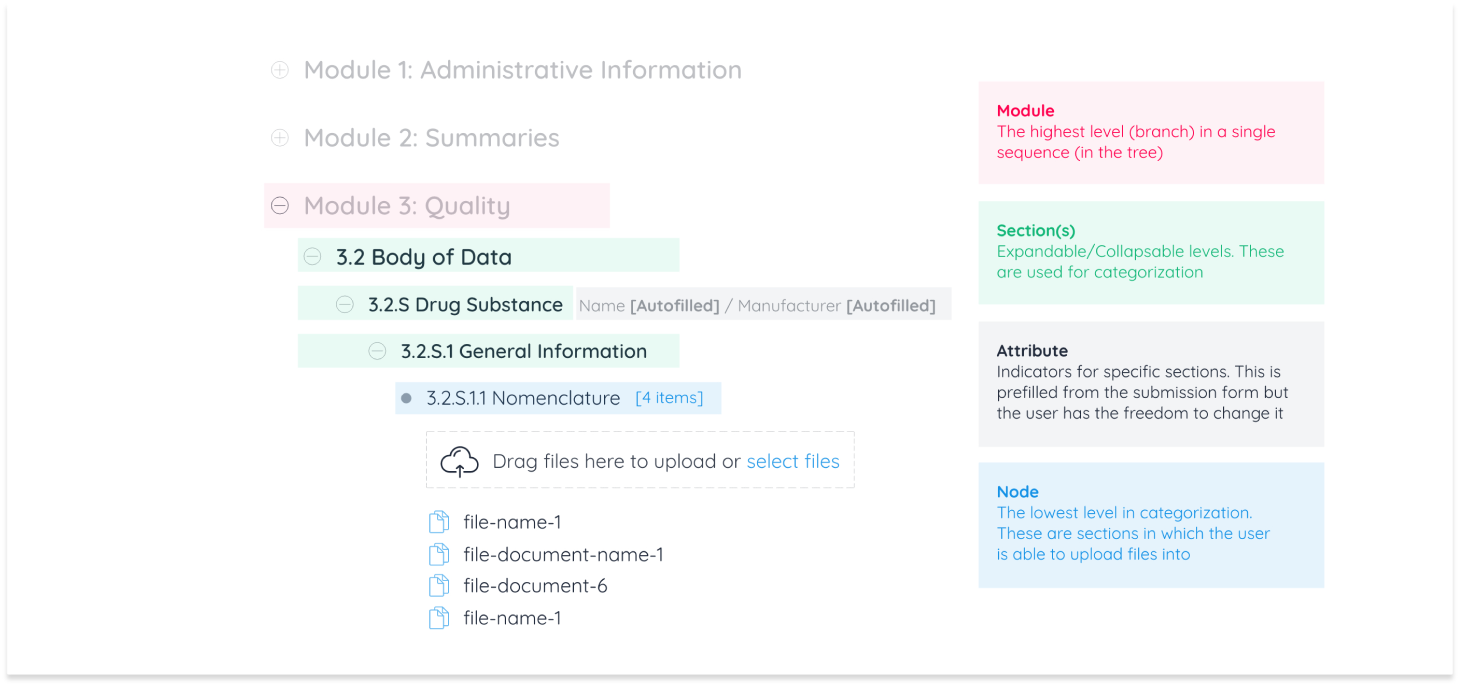
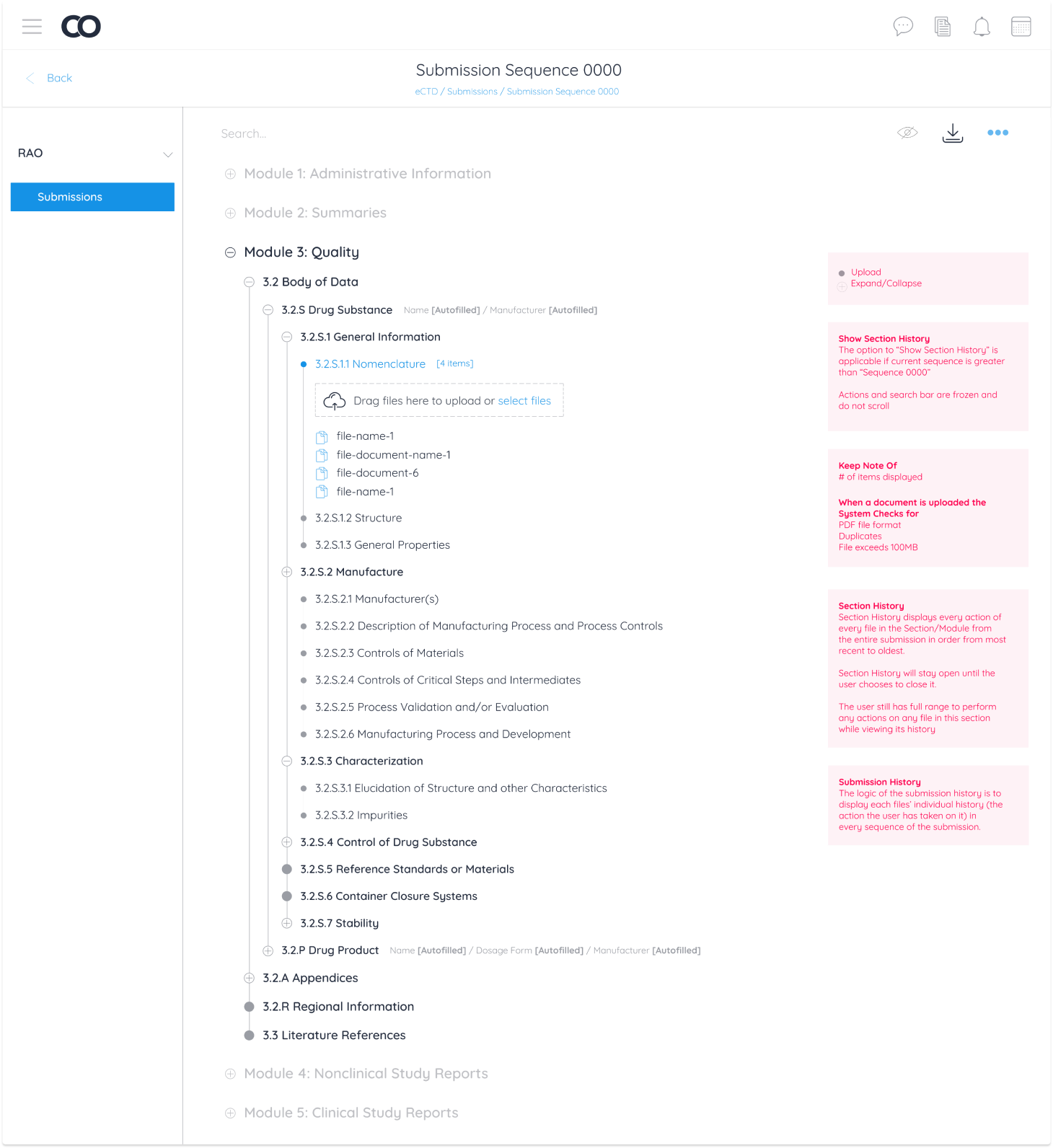
Due to the industry specific nature of this module, functionality was categorized into industry requirements and product requirements.
Pharmaceutical companies conduct all necessary testing and quality assurance on a new drug. All documentation from testing is then uploaded and sent to the targeted geographical region to accept, reject, or request edits on documentation. In the case of requesting edits for a new drug to be accepted, there are 3 operations regions are authorized to request: Replacing, Appending and Deleting a document from the eCTD module.
These 3 operations were categorized as “Business Requirements” for Regulatory Affairs Officers (RAO) alongside document management operations any Continuum user can perform.
Replace
Append
Delete
Upload
View PDF
Download
Delete
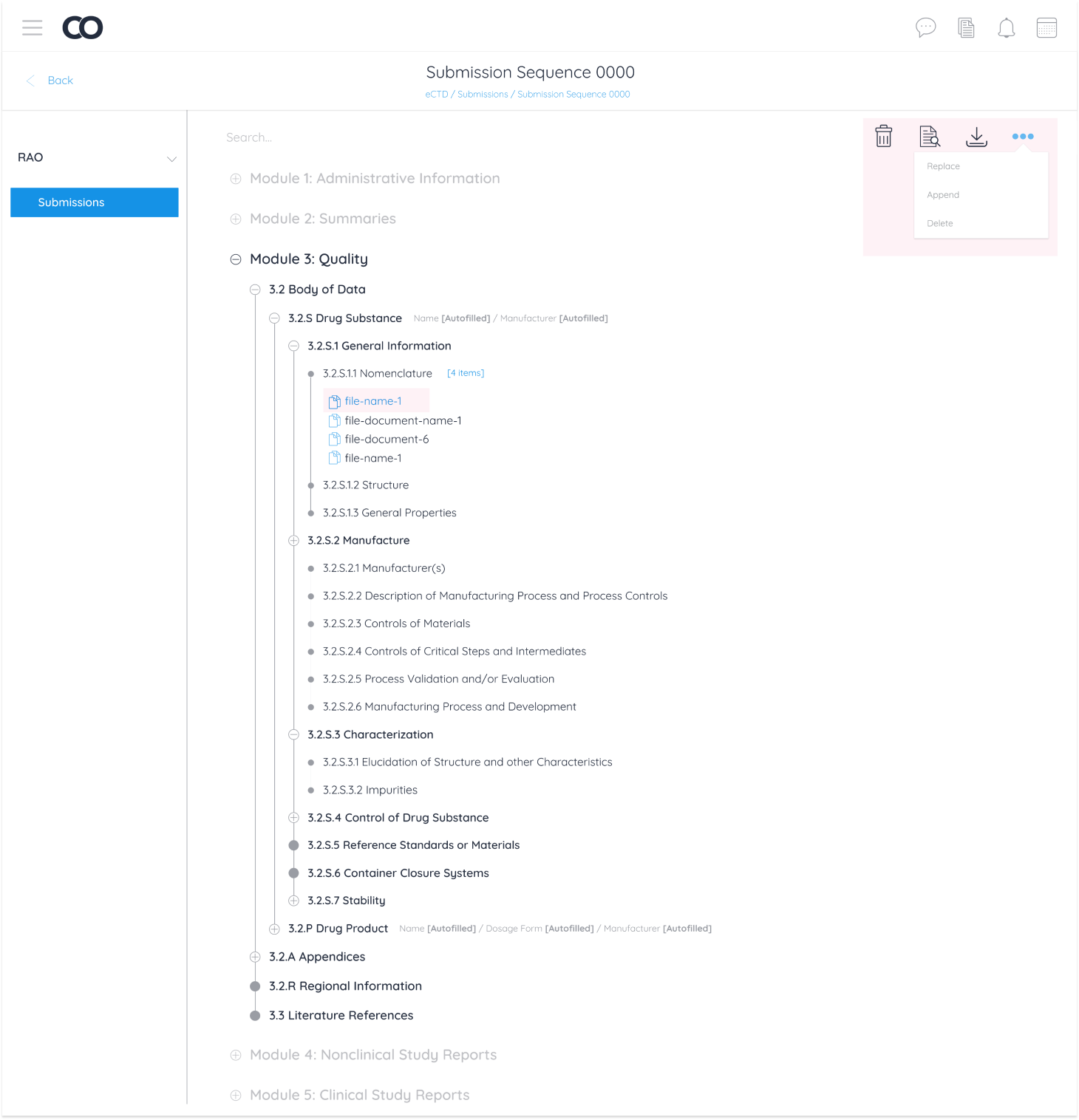
I then created a breakdown of interactions to display every action a user can perform

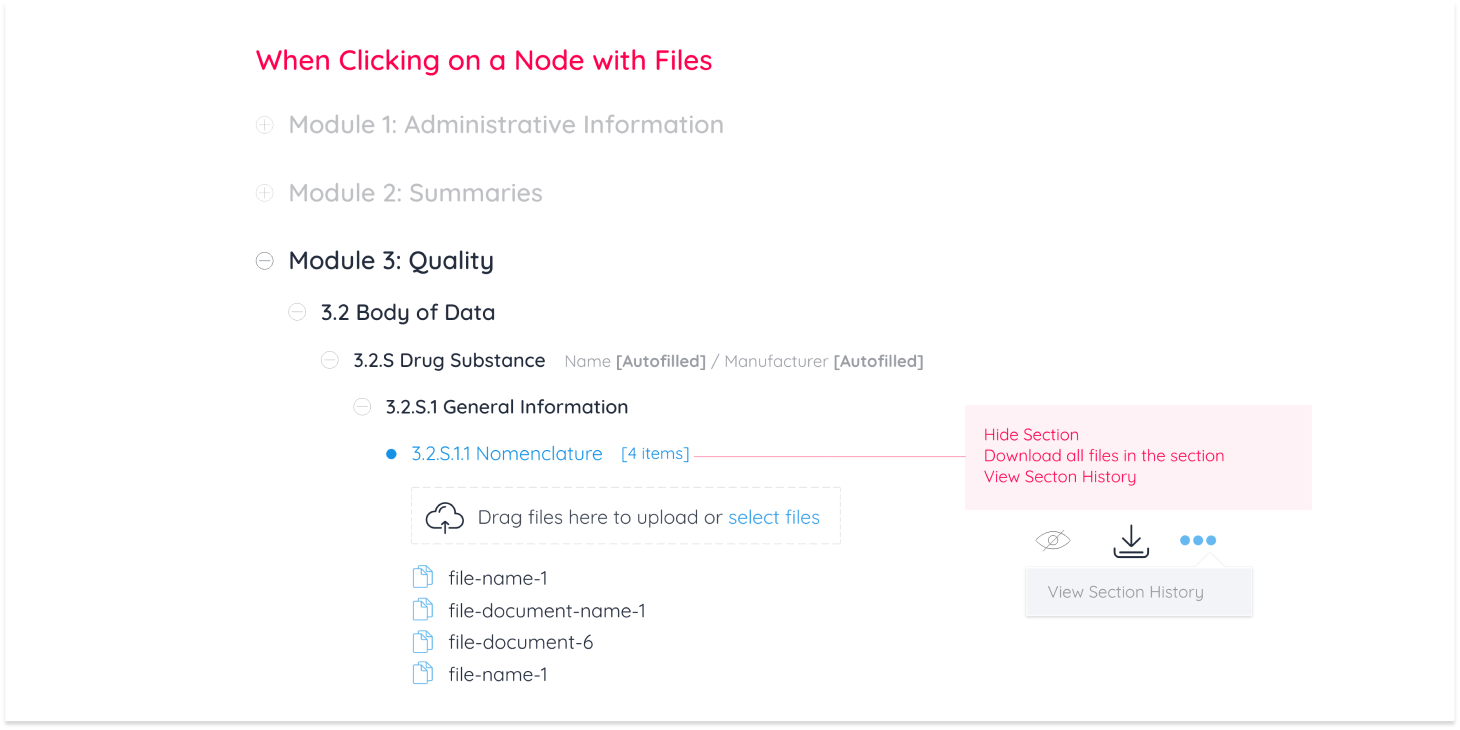
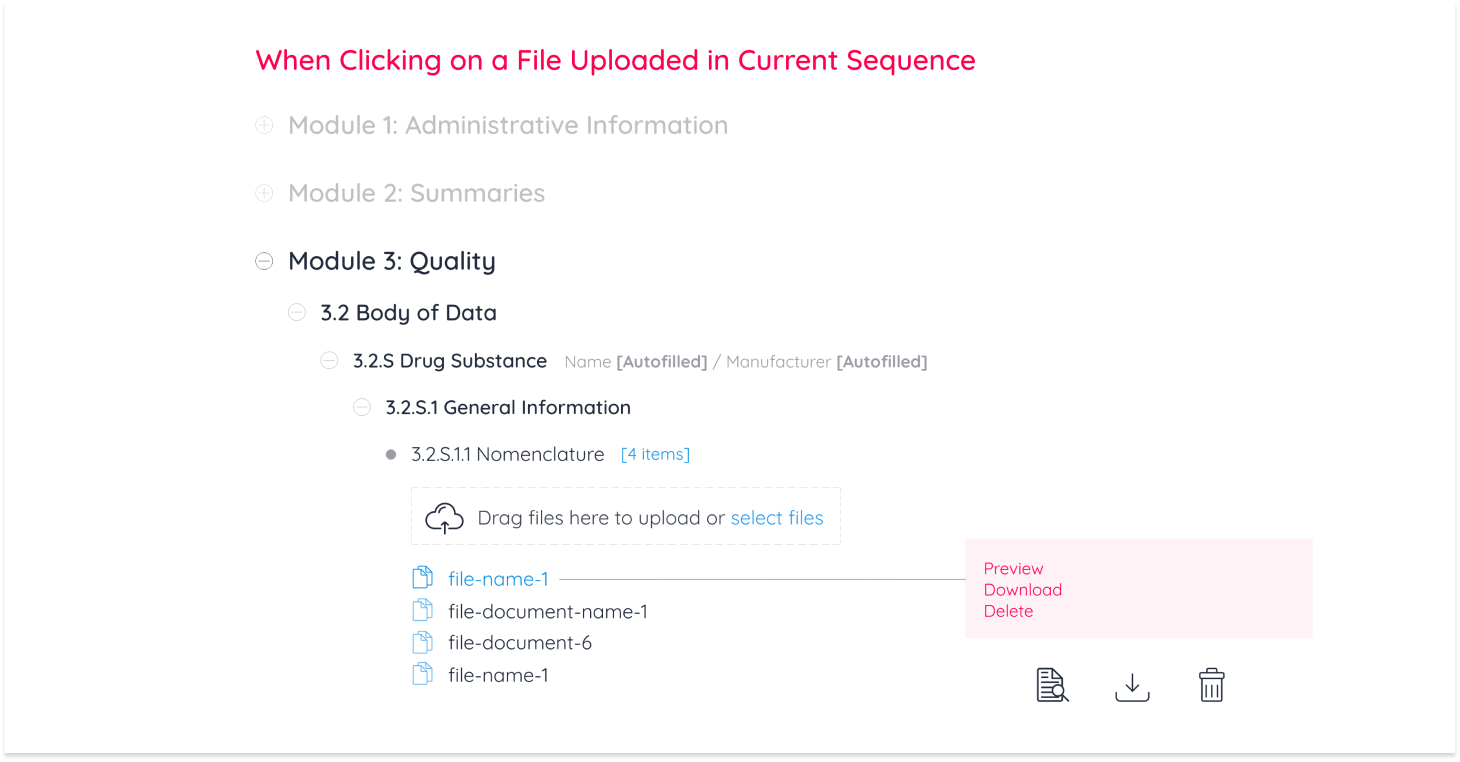
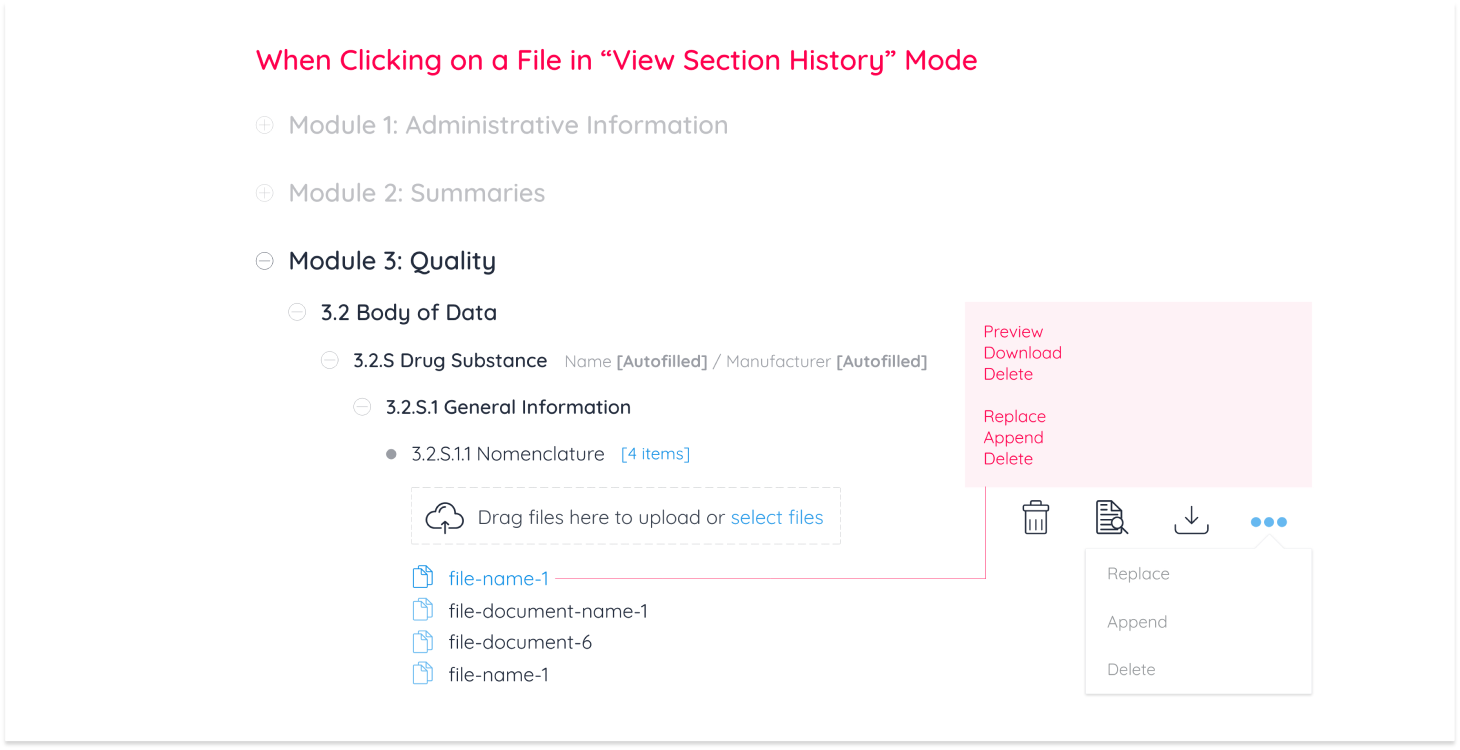

While user-to-system workflows gave a bird’s eye view of how the module functions, prototypes were made for the purpose of displaying document management operations to the development team and for usability testing. After 4 rounds of testing with industry experts, designs were finalized and documented.